Standardization of Siddha Herbal Formulation - Vaasathi Kashayam According to PLIM Guidelines
Abstract
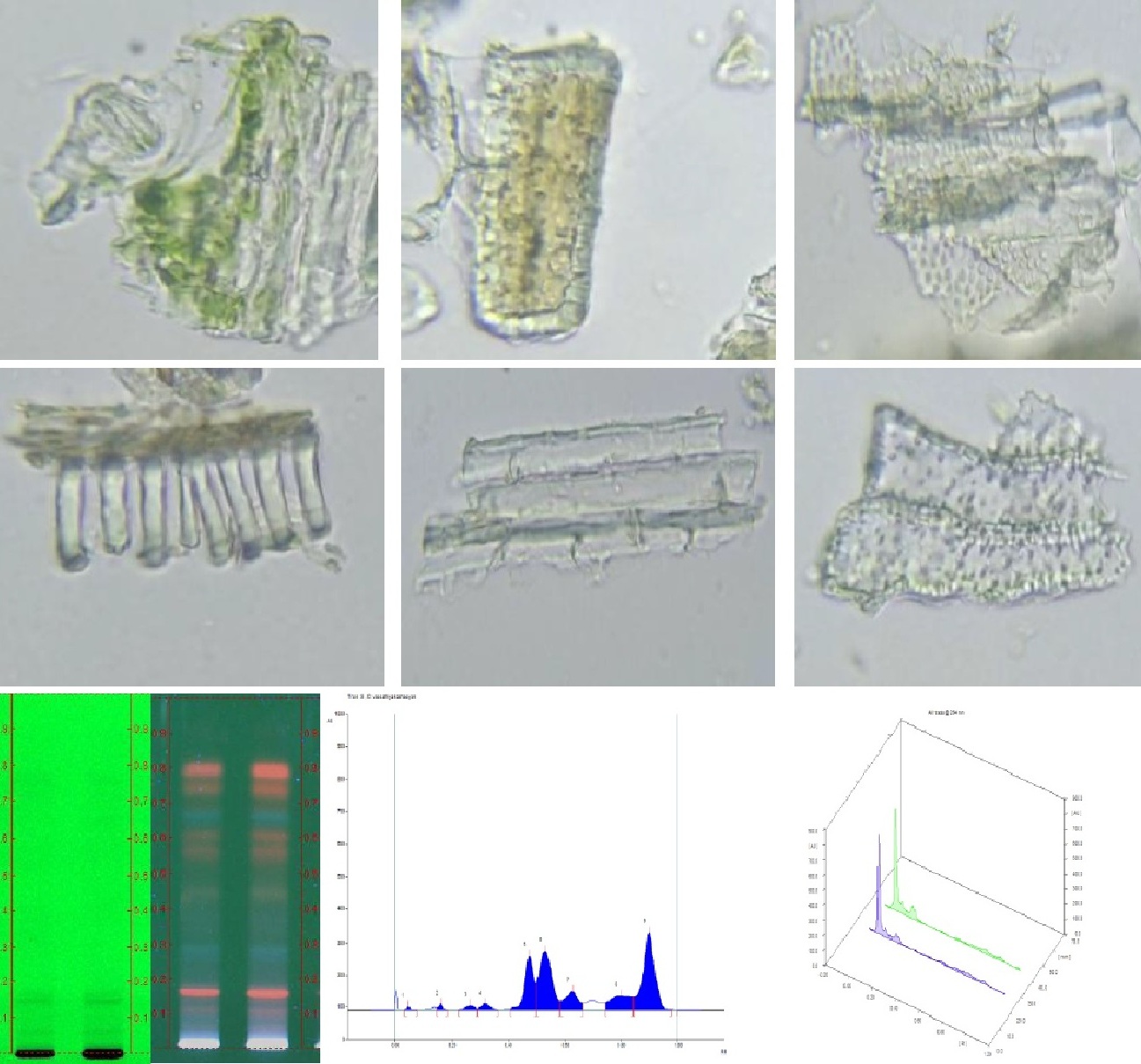
Despite the fact that the global market for herbal and traditional medicines has surged, the rise in demand has brought about a threat for adulteration/substitution of raw drugs, clamoring consumers' faith into skepticism with the ensuing implications. To evaluate and avoid substandard herbal medicine manufacture, the Indian government has taken stringent measures, notably the establishment of PLIM (Pharmacopeial Laboratory of Indian Medicine - Protocol for testing AYUSH pharmaceuticals). The purpose of this research is the standardization of Siddha herbal decoction formulation 'Vaasathi kashyam' encompassing the leaves of Justicia adhatoda.L (Acanthaceae) and dry fruits of Vitis vinifera.L (Vitaceae) procured from the classic Siddha text 'Agathiyar 2000' specifically indicated for systemic hypertension (Raththa kothippu noi). The physicochemical parameters, phytochemical analysis, and powder microscopy of Vaasathi kashayam revealed an acidic pH and demonstrated the presence of phytochemicals such as tannins, phenols, terpenoids, alkaloids, flavonoids, and carbohydrates. HPTLC fingerprinting illustrates phytochemical spikes. Furthermore, the number of heavy metals, pesticide residues (organochlorine, organophosphorus and pyrethroids) and aflatoxins in VK was trace (BQL) or nonexistent, indicating its safety for therapeutic use. The total aerobic bacterial count and yeast/mold growth reported no growth/colonies, implying that the sample is free of aerobic microorganisms. Standardization of VK entailed authenticating along with evaluating the safety and quality of the prepared VK sample. These standardized characteristics could be deployed as a reference standard to guide subsequent VK qc evaluations.
Downloads
Copyright (c) 2024 International Journal of Ayurveda and Pharma Research

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.






