Management of Grade III Internal Hemorrhoids by HAL (Haemrriodal Artery Ligation) and Tikshna Kshara Application
Abstract
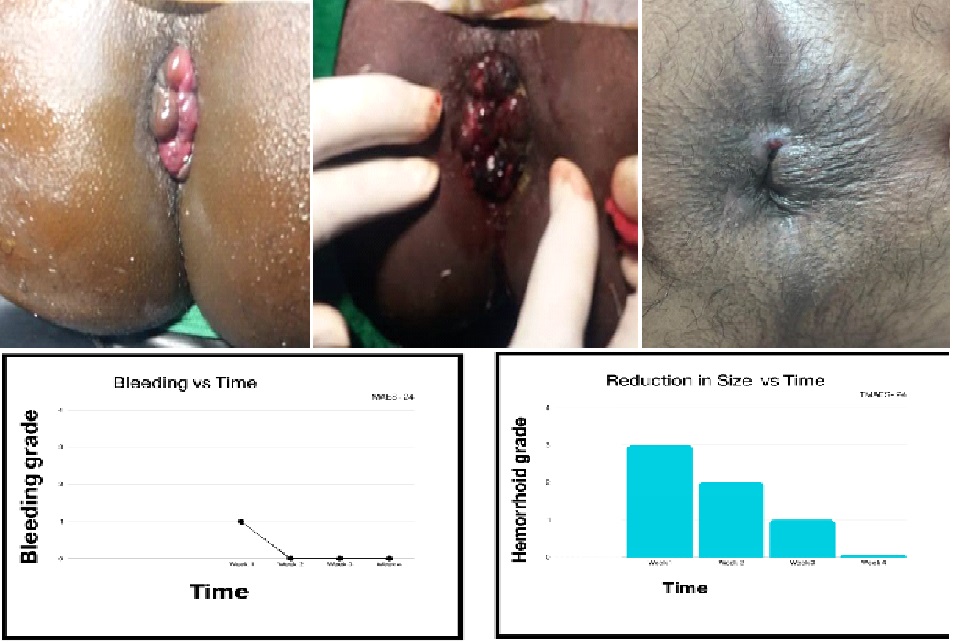
This study aims to standardize a hybrid procedure to be used in internal Hemorrhoids (Arsha) without complication of post-operative bleeding and severe pain experienced by patients in other forms of surgical interventions. Methodology: A patient diagnosed with Grade III hemorrhoids visited outpatient department and after thorough discussion, evaluation and physical/local examination was advised to undergo Tikshana Kshara therapy along with HAL for redressal of symptoms presented as bleeding per rectum. The patient was sent for routine pre surgical investigations and pre-anesthetic checkup. A surgical plan was devised for the case using Vicryl 2-0 for HAL and Tikshana Apamarga Kshara for application on pile mass. A half slit proctoscope was used for visualization of each pile mass. Suture of 8 shape was taken at the base of each pile mass for ligation with Vicryl 2-0 and Tikshana Kshara was applied after that on each pile mass for 40 sec one after the another. Khasra was washed with Nimbu Swaras to prevent Atidagdta by Kshara. There was minimal bleeding at the time of suturing and colour of the pile masses changed immediately to Pakva Jamunphala varna. A standard Kshara prepared using Apamarga, Suddha Varga and Chitraka was used for application. Patient was kept under observation for post-operative care. Efficacy was assessed after 15 days of the procedure. Results: The procedure produced encouraging results with significant reduction in size pile masses. There was no post-operative pain or constipation. There was minimum to no post-operative bleeding or stenosis. Conclusion: This hybrid combination by usage of Vicryl 2-0 and Tikshana Kshara application shows promising results in management of G-III hemorrhoids with no to minimal complication and post-operative care. Patient can immediately get back to work with some precautions.
Downloads
Copyright (c) 2024 International Journal of Ayurveda and Pharma Research

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.






