Standardization and Quality Assessment of Drakshavaleha: Insights from Three Marketed Brands
Abstract
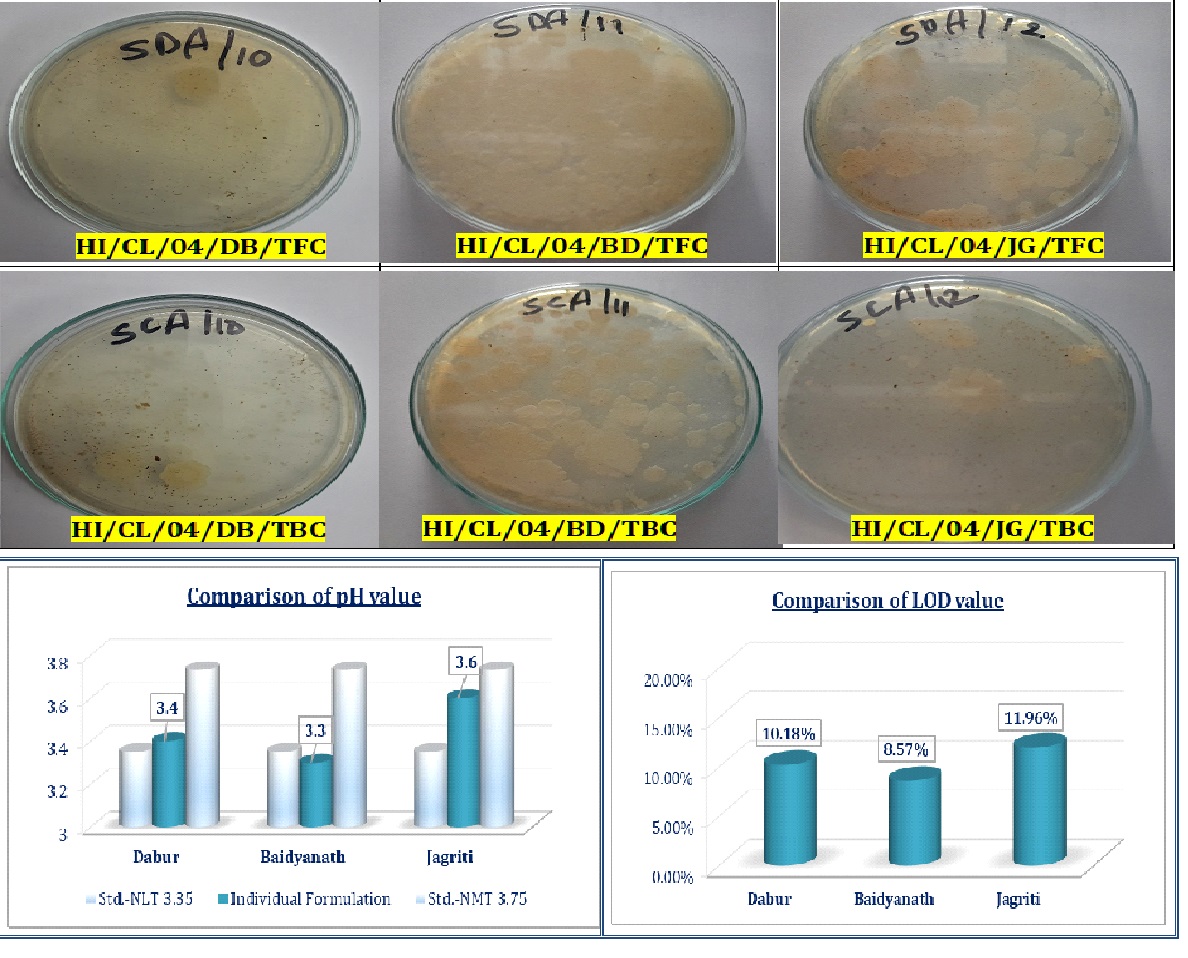
Drakshavaleha, a traditional Ayurvedic formulation, is widely used for its therapeutic benefits in treating respiratory and digestive ailments. This study aims to standardize and evaluate the quality of Drakshavaleha from three different marketed brands through a comprehensive assessment of their physicochemical and pharmacognostical properties, alongside an estimation of toxic heavy metals content and microbial contamination, and phytochemical profiles. Materials and Methods: Three brands of Drakshavaleha were meticulously assessed through a range of tests, including organoleptic analysis, physicochemical evaluations, heavy metal content analysis, microbial contamination assessments, and qualitative phytochemical screenings. Standardized protocols and methodologies from pharmacopoeias were used for all evaluations to ensure reliable and consistent results. Results and Discussion: The findings indicated notable variations in the evaluation parameters of the different brands. Physicochemical and Pharmaceutical analysis revealed disparities in pH levels and total reducing sugar levels. Heavy metal testing and microbial contamination levels were within acceptable ranges for all samples. The qualitative phytochemical screening identified differences in the concentration of key active ingredients, potentially impacting therapeutic efficacy. Conclusion: This study underscores the importance of standardization in maintaining the safety and efficacy of Ayurvedic formulations. By identifying variations in quality and safety parameters, it provides critical insights for manufacturers to improve their production processes. Ultimately, this research advocates for enhanced quality assurance practices to uphold the integrity of Ayurvedic medicine in the global market.
Downloads
Copyright (c) 2024 International Journal of Ayurveda and Pharma Research

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.






