A Classical Review on Pharmaceutical and Analytical Study of Abhrak Bhasma
Abstract
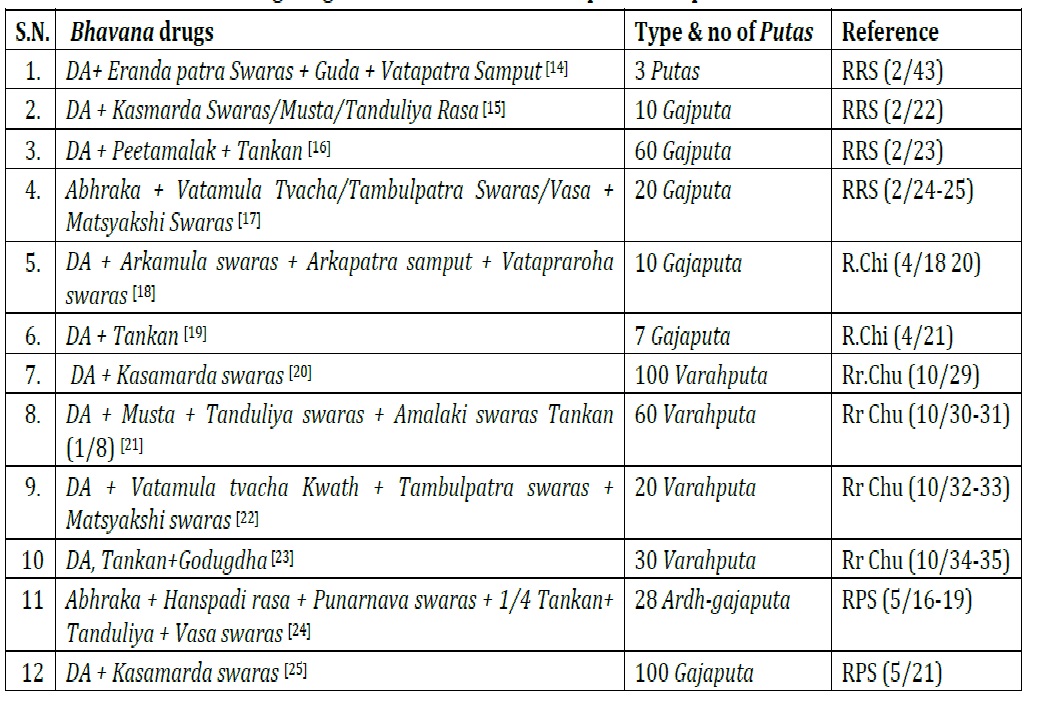
Abhrak bhasma is an important traditional preparation which possesses higher therapeutic value and is widely prescribed for skin disorders, respiratory ailments and other chronic conditions. In order to ensure the quality of Abhrak Bhasma, needs a critical study of its pharmaceutical aspects such as quality of raw Abhraka, Shodhan process, Dhanyabhrak Nirman and Maran of Abhraka as well as Abhrak Bhasma tested on the basis of organoleptic characteristics and classical Bhasma pariksha. Hence the present study reviewed classical literature on Abhrak Bhasma. Aim and Objectives: To review classical literature on Pharmaceutical study of Abhrak Bhasma with the objective to evaluate pharmaceutical aspects of Abhrak Bhasma critically on various factors such as Shodhana process, Marana process, temperature pattern, no. of Putas etc. Methods and Materials: All the classical literature in Rasagrantha, Samgraha Grantha, textbooks of Ayurveda and Rasashastra regarding pharmaceutical and analytical studies of Abhrak Bhasma. Observation and Result: The classical literature revealed that the pharmaceutical studies of Abhrak Bhasma like Shodhana, Dhanyabhraka, and Marana were done with specific guidelines in order to obtain the quality of Bhasma. Most of the literature followed Triphala Kwatha as a Shodhana media and Kanji for Dhanyabhraka process. The range of Puta Pramana observed was from 3 to 100 to ensure the quality of Bhasma. Abhrak Bhasma tested on the basis of organoleptic characteristics and classical Bhasma pariksha. Conclusion: In this review, the classical guidelines of pharmaceutical study of Abhraka Shodhana, Dhanyabhraka, and Marana were followed which set some important features in terms of temperature pattern, number of Puta etc. Further repeated studies on Abhrak Bhasma will help to establish standard operating procedure of it as well as fix its analytical parameters.
Downloads
Copyright (c) 2023 International Journal of Ayurveda and Pharma Research

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.






