Pharmaceutical Standardisation and Physicochemical Analysis of Krimighatini Vati
Abstract
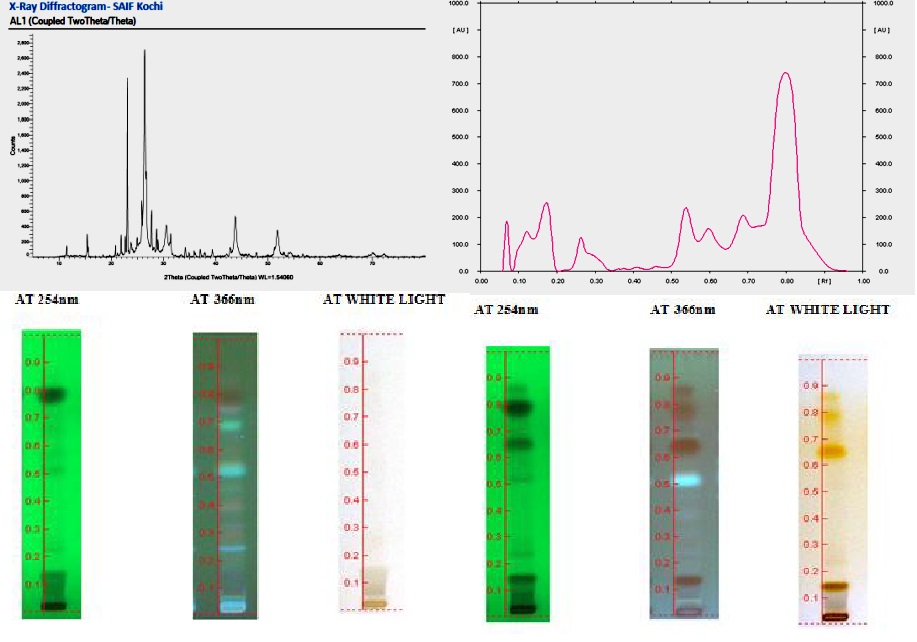
Standardization is the process for the establishment of standard for a particular or drug. Standardization in Ayurvedic formulation mainly deals with ensuring standards for the quality and purity of raw materials, quality control during the drug manufacturing process, production of a good quality finished product and storage and distribution to maintain the quality of the final product. Standard of an Ayurvedic product can be assessed only by analyzing the analytical parameters of raw drugs, drugs after preprocessing and the finished products. Krimighatini vati explained in Rasendra chintamani is an antihelminth preparation explained in Rasendra Chintamani krimirogadhikara. It contains Parada- 1part, Gandhaka- 2 parts, Ajamoda -3 parts, Vidanga bheeja- 4 parts, Palaasha bheeja- 5parts, and Kaarsakara beeja- 6 parts. It is similar to Krimimudgara rasa that is available in market but differs in proportion of Palaasha beeja and Karaskara beeja. Present study aims to standardize Krimighatini vati after preparing the medicine according to the method explained in Ayurveda samhithas. Preprocessing of the Gulika includes, Shodhana of Hingula, Gandhaka and Karaskara beeja according to the methods mentioned in Rasasastra books. Parada was extracted from Hingula by Ordhwapaathana vidhi. It was then triturated with Shuddha Gandhaka to get Kajjali. Fine powders of other drugs were mixed with Kajjali and rolled into pills with Honey. Study observed the physicochemical parameters of individual drugs and validated the Gulika by HPTLC method. Analysis of Kajjali was done using XRD so as to prove the complete formation of Kajjali. Pharmaceutical standardization helps in reproducibility of drug paving way for more studies on toxicity and efficacy of drug.
Downloads
Copyright (c) 2023 International Journal of Ayurveda and Pharma Research

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.






