Pharmaceutical Standardization and Physicochemical Analysis of Arogyavardhini Vati
Abstract
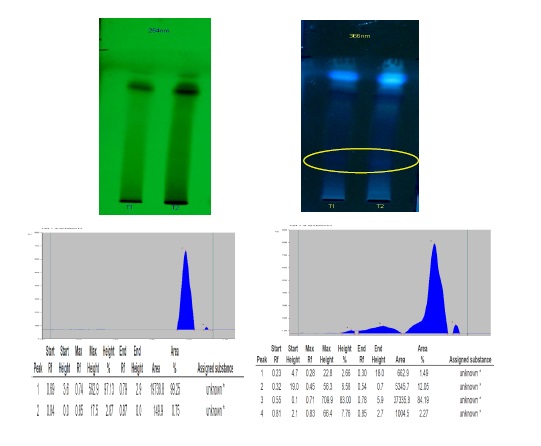
It is very crucial to know the physicochemical properties of drugs during the development of pharmaceutical products. Drugs are on compulsion to assess their compatibility of active substances, excipients and medicinal products with established standards by the pharmaceutical laws. Characterization of these active pharmaceutical ingredients (APIs) helps in improving the quality parameters of all raw materials used during the manufacturing process of pharmaceuticals and also in the final products. Maintaining quality standards of the drugs is the need of the hour in this era of increasing demand for indigenous medicines. Due to the absence of reference standards, standardisation of many of compound formulations is lagging behind. Ayurveda is one of the oldest medical science that has been serving the community since centuries. Arogyavardhini vati is one of the most important Ayurvedic formulation that is advised by the Ayurvedic scholars for liver disorders since centuries. Although, being administered by a vast community of Ayurvedic practitioners and from a very long period with multiple benefits, there were no many studies that are available on the physicochemical analysis and standardization of Arogyavardhini vati. Present study evaluated the physicochemical properties of Arogyavardhini vati and standardized. Arogyavardhini vati prepared by the Ayurvedic classical method complies with the standard parameters as mentioned in Ayurvedic Pharmacopeia of India. Present study observed that the analytical parameters and the pharmaceutical parameters for Arogyavardhini Vati were validated by HPTLC method and can be considered as the standard drug.
Downloads
Copyright (c) 2022 International Journal of Ayurveda and Pharma Research

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.






