Real-World Experience of Hemoxin R Plus (Nikosan K Plus): Retrospective Analysis on 150 Patients
Abstract
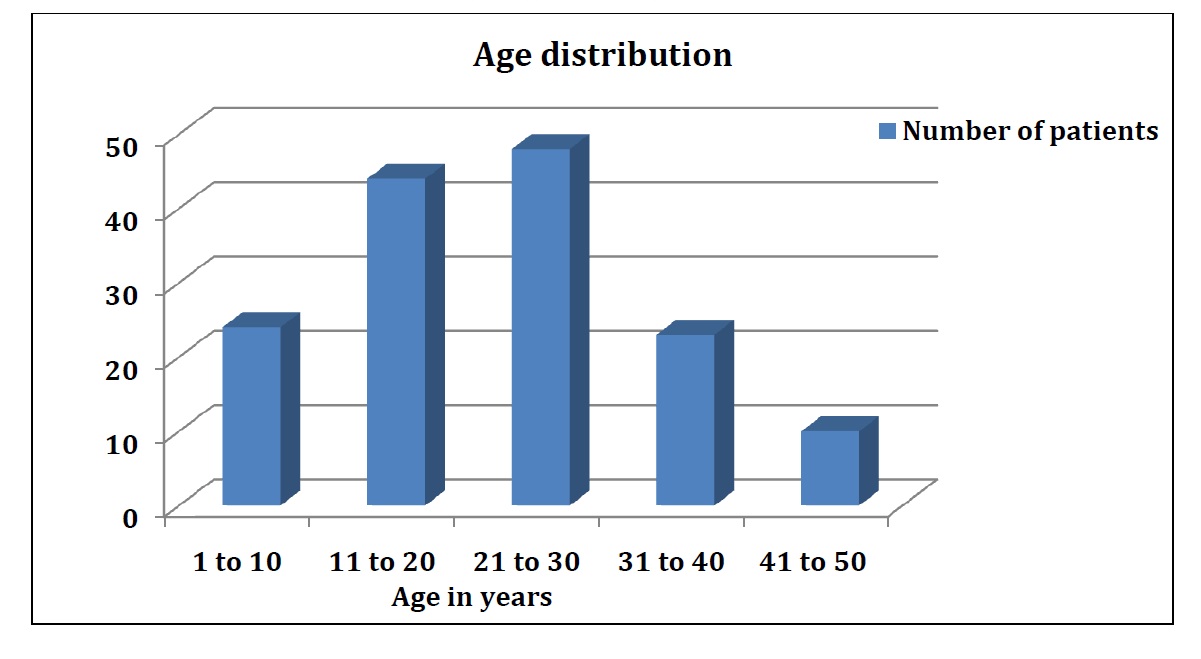
Sickle cell disease (SCD) is amongst the most common genetic hematological disorders. Hand-foot syndrome (swelling), pain and anemia are some of the very common complications of the disease. In Sickle cell anemia, the number of healthy RBCs decrease which results in reduction of oxygen in the tissues. Majority of the SCD patients are from low socio-economic strata and can barely afford costly treatment modalities. Retrospective analysis was done on 150 patients who consumed a proprietary Ayurvedic medicine, Hemoxin R Plus (Nikosan K Plus). Objectives: Sickle cell anemia impacts quality of life of patients which include pains including joint pain, abdominal pain, and total body pain. It also leads to breathlessness, weakness or fatigue and hence difficulties in doing daily chores. Our aim was to evaluate safety and efficacy of Hemoxin R Plus, an Ayurvedic medicine in improving quality of life in patients having SCA. Materials and Methods: This was a retrospective analysis. Hospital records of the patients were used and reviewed for the analysis. The doctors who treated the patients collected the data from the medical records department. Wilcoxon signed rank test was applied for analysis. Parameters related to the quality of life were studied. The parameters considered were pain (whole body, abdominal, limbs/joints, back), fatigue, breathlessness, difficulty in doing daily activities and absenteeism (school/job). Results: For every parameter considered for analysis, the probability value (p value) was found to be <0.05, confirming the statistical significance in reduction of symptoms. Hemoxin R Plus was found to be safe in the dose administered, as there were no adverse events reported. Conclusion: Capsule Hemoxin R Plus can be used for of the management of sickle cell anemia in pain reduction and in improving the quality of life.
Downloads
Copyright (c) 2022 International Journal of Ayurveda and Pharma Research

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.






