Standardisation of Vilwadi Leha - A Process Validation Based on Total Solid Method
Abstract
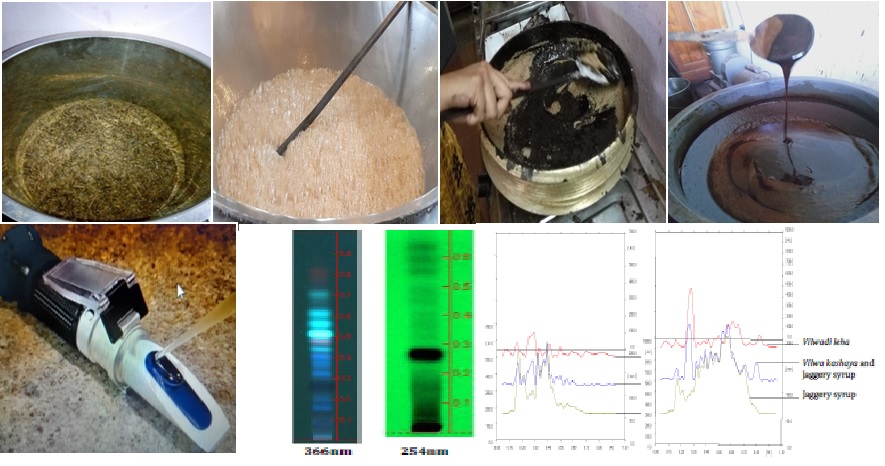
Standardisation can be defined as a process of enforcing a level of consistency or uniformity in pharmaceutical preparations which are operated within the selected environment. Vilwadileha is a well-known Ayurvedic polyherbal formulation used for the management of various diseases such as Chardi, Arochaka, Praseka, Agnimandhya etc. It is described in Sahasrayoga leha prakarana. Aim: The objective was to develop the standard manufacturing procedure (SMP) and quality standards of Vilwadi leha based on total solid method. Total soluble solids content of a solution is determined by the index of refraction using either hand refractometer or Abbe refractometer. Brix is the term used when a refractometer equipped with scale, measures total soluble solids of a pure aqueous sucrose solution by the aid of refractive indices at 20°C and percentage by mass. Materials and methods: In present work, VL was prepared in three batches as per standard guidelines mentioned in AFI Vol 2. Results: Samyak leha paka lakshanas observed was compared with brix value and fix the brix value of VL prepared in 3 Batches. It took 10.40hr to completion of preparation. Conclusion: The current study opens a new concept to standardise Avaleha. This is a trial work to standardise the Leha paka with total solids analysis method or brix value. Brix for each preparation may differ based on sucrose concentration. It was found that Leha prepared with TS between 75 and 77 had parameters similar to Samyak Leha paka. Attempts were also made to develop analytical profile of final product.
Downloads
Copyright (c) 2023 International Journal of Ayurveda and Pharma Research

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.






