Formulation and Evaluation of Orodispersible Tablet Containing Combination of Phosphodiesterase-5 Inhibitor and Herbal Aphrodisiac
DOI:
https://doi.org/10.47070/ijapr.v11i2.2700Keywords:
Orodispersible Tablets, Taste masking, Solubility enhancement, Sildenafil citrate, Ginseng, Erectile dysfunction.Abstract
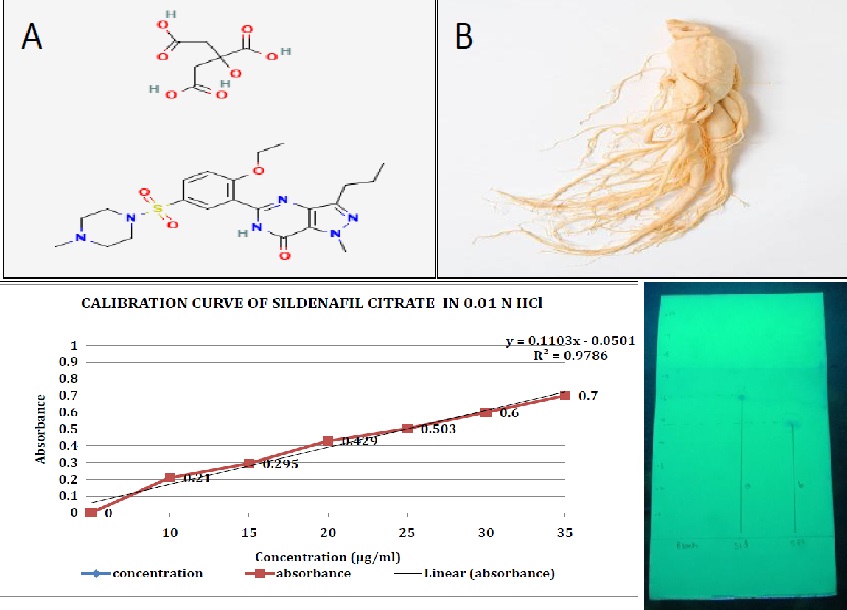
Purpose: Sildenafil citrate is widely used drug for the treatment of Erectile Dysfunction (ED) and Ginseng is a natural aphrodisiac reported to benefit this condition. The objective of the present study was to develop orodispersible tablets (ODTs) containing combination of Sildenafil citrate and Ginseng extract to improve the bioavailability, reduce the dosing frequency and thereby maintaining the therapeutic efficacy of the drug. Methods: The ODTs were prepared using superdisintegrants such as Croscarmellose sodium (CCS), povidone, and sodium starch glycolate (SSG) at varying concentrations (2%, 4% and 6%) by direct compression. The bitter taste of Sildenafil citrate was masked by Doshion resin. The optimized formulation based on least disintegration time (DT) was chosen to reformulate using sublimating agents such as camphor, menthol or thymol at varying concentrations (1%, 2%, 3%) to further reduce the DT. The compatibility of drug with excipients was investigated and the prepared formulations were evaluated for pre and post-compression parameters. Results: The post-compression parameters such as weight variation, hardness, friability, DT and in-vitro drug release was found within specified limit. The formulation with camphor (2%) had DT of 12 sec and drug release >90% within 5 min hence was considered as optimized formulation. The accelerated stability study and kinetics modelling was performed for optimized formulation. Conclusion: The formulated Sildenafil citrate and Ginseng ODT’s were found to be promising formulation with quicker DT and drug release which will eventually have higher bioavailability and better efficacy along with averting the issues of swallowing and improving patient compliance.
Downloads