Analytical Profile of Kanchanara Guggulu Tablets Prepared as per References in Sharangadhara Samhita and Bhaishajya Ratnavali
Abstract
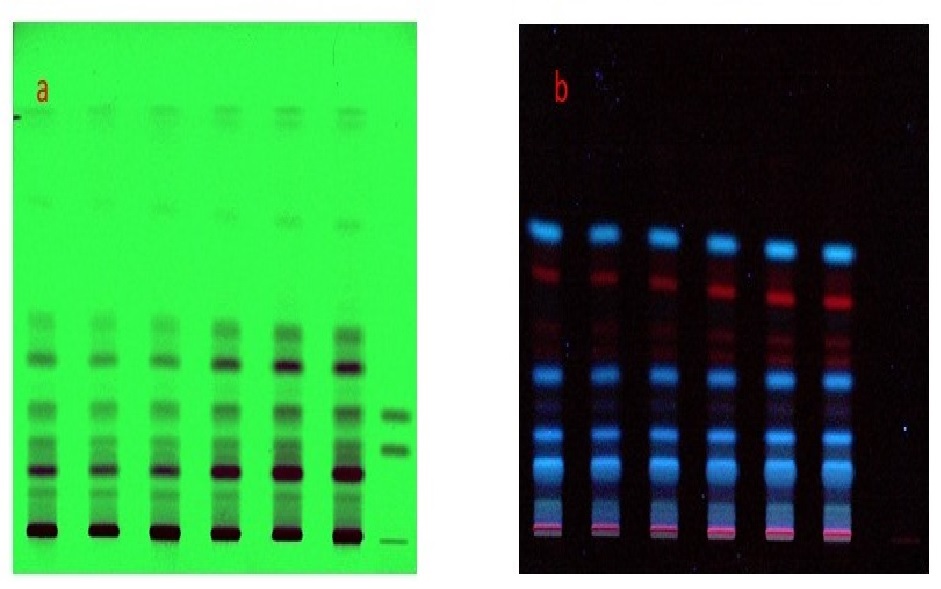
Marketing of Guggulu, an exudate obtained from the plant Commiphora mukul and its preparations is a great concern of different ayurvedic pharmaceutical houses due to its adulteration, substitution and non-availability of genuine samples. Standardization of raw drugs and formulations with modern analytical tools increase their scope, acceptance and scientific validity. In the present study, an attempt was made for the physicochemical analysis of Kanchanara guggulu tablets (having Guggulu as the major ingredient) prepared as per references in Sharangadhara Samhita and Bhaishajya Ratnavali to develop an analytical profile of the formulation. The study was based on the standard analytical parameters proposed by API and PLIM. Six samples of tablets were prepared as per two references (three for each reference) which were available in market following the same manufacturing procedures. The study comprised of three stages- pharmacognosy of raw drugs, pharmaceutical work and analytical study. The analysis was done using the parameters like organoleptic evaluation, weight variation, hardness, friability, disintegration time, ash values, extractive values, loss on drying, pH, HPTLC and microbial contamination and an analytical profile was developed as per both references.
Downloads
Copyright (c) 2022 International Journal of Ayurveda and Pharma Research

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.






